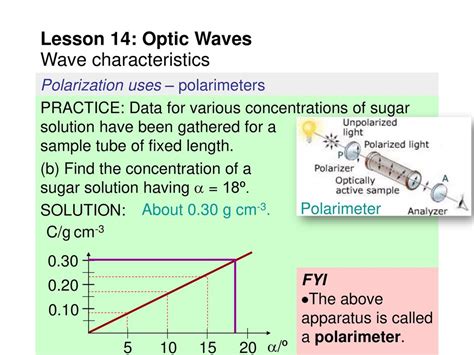
polarimeter reaction flask|polarimetry chemistry pdf : purchasing A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the laboratory component of the course. . the products are racemic unless a single enantiomer of a chiral co-reactant or catalyst is involved in the reaction . Role Promocional Show 2021 Washington Brasileiro 41.901 plays. A Dani Senta Promocional Show 2021 Washington Brasileiro 87.540 plays. Dois Bebim (Live 3) .
{plog:ftitle_list}
Resultado da Vazados Martina Oliver photos & videos. EroMe is the best place to share your erotic pics and porn videos. Every day, thousands of people use EroMe to enjoy free photos and videos. Come share.
describe the features and operation of a simple polarimeter. calculate the specific rotation of a compound, given the relevant experimental data. A pure sample of the naturally-occurring, chiral compound A (0.250 g) is dissolved in acetone (2.0 mL) and the solution is placed in a 0.5 dm cell. Three polarimetry readings are .
Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer C) pressure measurement D) none of the above E) all of the above, A gas chromatograph can be used to determine the relative amounts of reactants and products by, Give the characteristic of a first .A polarimeter is an instrument used to determine the angle through which plane-polarized light has been rotated by a given sample. You will have the opportunity to use a polarimeter in the laboratory component of the course. . the products are racemic unless a single enantiomer of a chiral co-reactant or catalyst is involved in the reaction .Identify the methods used to monitor a reaction as it occurs in the reaction flask: Polarimeter Scetrometer Pressure measurement : Give the charateristics of the first order reaction having only one reactant: The fate of the reaction is directly proportional to the concentration of .
Identify the methods used to monitor a reaction as it occurs in the reaction flask. a. polarimeter b. spectrometer c. pressure measurement. Rate law. rate of a reaction is directly proportional to the concentration of each reactant. Rate law expression. Rate = k[A]^n.Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask., T/F: The half life of a first order reaction is dependent on the initial concentration of reactant, T/F: The half life of a second order reaction is not dependent on concentration and more.Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer C) pressure measurement D) all of the above E) none of the above, Give the characteristic of a first order reaction having only one reactant. A) The rate of the reaction is not proportional to the .
Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask., Given the following balanced equation, determine the rate of reaction with respect to [NOCl]. 2 NO(g) + Cl2(g) → 2 NOCl(g), Given the following balanced equation, determine the rate of reaction with respect to [H2]. N2(g) + 3 .By reducing the path length of the sample cell from 100 mm to e.g. 2.5 mm or reducing the concentration of the sample, the result will be compatible with the measuring range of the polarimeter. In order to determine the specific rotation of a substance, the MCP polarimeter can use a shorter sample cell than 100 mm.Study with Quizlet and memorize flashcards containing terms like methods used to monitor a reaction as it occurs in the reaction flask, FIRST order RX having only one reactant., ZERO order reaction having only one reactant. and more. . polarimeter spectrometer pressure measurement. FIRST order RX having only one reactant. rate of the reaction .
Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask A) spectrometer B) polarimeter C) pressure measurement D) none of the above E) all of the above, Write a balanced reaction for which the following rate relationships are true. Rate = 1/2 ∆[N2] /∆t = ∆[O2]/∆t = 1/2 ∆[N2O]/∆t A) 2 N2 + .
polarimetry test
The methods used to monitor a reaction as it occurs in the reaction flask are d) all the above. A polarimeter is an analytical hardware component that measures the polarization of light and is used to analyze the optical activity of chemicals. Optical activity refers to the ability of a material to rotate polarized light and the extent to which such a phenomenon occurs.Study with Quizlet and memorize flashcards containing terms like 1) Given the following proposed mechanism, predict the rate law for the overall reaction. 2NO2+Cl2--->2NO2Cl (overall) Mechanism 1) NO2+Cl2---> NO2Cl+CL (slow) 2)NO2+Cl--->NO2Cl (fast), 2) Identify the methods used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer . In measuring optical rotation, plane-polarized light travels down a long tube containing the sample. If it is a liquid, the sample may be placed in the tube as a pure liquid (its is sometimes called .Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask A)polarimeter B) spectrometer C) pressure measurement D) none of the above E) all of the above, A gas chromatograph can be used to determine the relative amounts of reactants and products by A) measuring the changes in .
Chemistry document from New Jersey Institute Of Technology, 2 pages, 1) Identify a method NOT used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer C) gas chromatograph D) stir rate 2) Write a balanced reaction for which the following rate relationships are true. Rate = - = = A) 2 N2Study with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask., Which of the following statements is FALSE? A) The average rate of a reaction decreases during a reaction. B) The half-life of a first-order reaction is dependent on the initial concentration of reactant. C) It is not possible to .
Scot Determining The Mechanism Of A Substitution Reaction Using Polarimetry experiment favors Sn2 Procedure For this experiment, a solution of 11 mL 90% L-Lactic acid and 15 mL 48% HBr needed to be prepared in a flask and then put .
Instruments used to measure the angle of rotation caused by passing polarized light through an optically active substance. Polarimeters are used in the chemical, pharmaceutical, and food industries for monitoring quality, purity, and concentration and indicating the progress of reactions and conversions.Study with Quizlet and memorize flashcards containing terms like The decomposition of dinitrogen pentoxide is described by the chemical equation 2 N2O5(g) → 4 NO2(g) + O2(g) If the rate of disappearance of N2O5 is equal to 1.40 mol/min at a particular moment, what is the rate of appearance of NO2 at that moment?, Given the following balanced equation, determine the . Polarimeter is the instrument used to determine the specific rotation of a compound. It consists of the following: monochromatic light source; polarizer, a prism that converts regular light into plane-polarized light; sample tube; analyzer, a prism through which the light leaving the sample is observed; see also optical rotationStudy with Quizlet and memorize flashcards containing terms like Identify the methods used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer C) pressure measurement D) all of the above E) none of the above, Give the characteristic of a first order reaction having only one reactant. A) The rate of the reaction is not proportional to the .
Study with Quizlet and memorize flashcards containing terms like This equipment is used to transfer solid chemicals from containers to transfer to other glassware such as beakers and flask A) Spatula B) Test tube holder C) Beaker D) Dropper, The proper procedure for crystallization is to allow the hot solution to cool to room temperature, then to chill the solution in an ice bath.Rate = 2 Δ[SO 2 ] t e. It is not possible to determine without more information. 2. Identify the methods used to monitor a reaction as it occurs in the reaction flask. a. polarimeter d. none of the above b. spectrometer e. all of the above c. pressure measurement 3. Write a balanced reaction for which the following rate relationships are true. We describe a high resolution laser polarimeter built from commodity optical components. The optical rotation angle is determined by measuring the phase difference between two harmonically .The methods used to monitor a reaction as it occurs in the reaction flask are d) all the above. A polarimeter is an analytical hardware component that measures the polarization of light and is used to analyze the optical activity of chemicals. Optical activity refers to the ability of a material to rotate polarized light and the extent to which such a phenomenon occurs.
Exercise \(\PageIndex{1}\) A pure sample of the naturally-occurring, chiral compound A (0.250 g) is dissolved in acetone (2.0 mL) and the solution is placed in a 0.5 dm cell.Chemistry: A Molecular Approach, 3e (Tro) Chapter 13 Chemical Kinetics Multiple Choice Questions 1) Identify the methods used to monitor a reaction as it occurs in the reaction flask. A) polarimeter B) spectrometer C) pressure measurement D) none of the above E) all of the above Answer: E Diff: 1 Page Ref: 13.2 2) Given the following balanced .
polarimetry practice
22 de fev. de 2024 · As apostas em tênis são uma forma de se envolver mais com o esporte. Para aqueles que estão começando, entender como funcionam as apostas é essencial. Inicialmente, deve-se conhecer os tipos de apostas disponíveis. Pode-se apostar no vencedor da partida, no número total de sets ou até mesmo nos pontos .
polarimeter reaction flask|polarimetry chemistry pdf